View Procedure
| Procedure Name | Health Certificate for EU countries | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
Required Documentation
Requirements
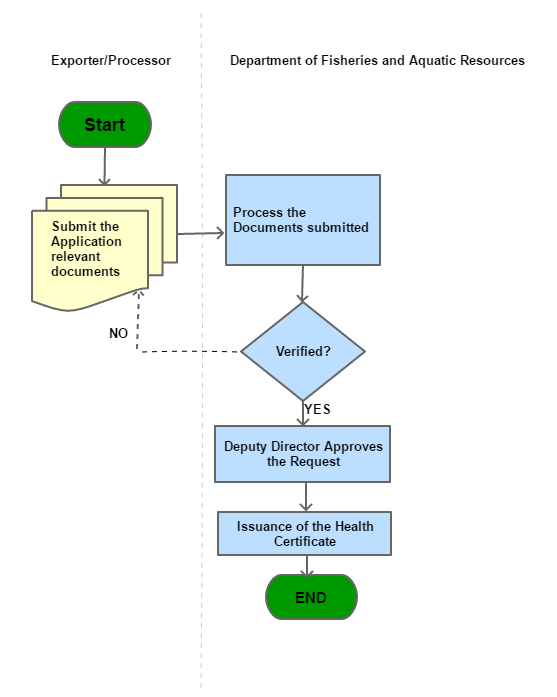
Process Steps
*If the shipment is canceled, the applicant returns the un-used health certificate with reasons for non-use for cancellation.
| |||||||||||||||||||||||
| Category | Export |
The following form/s are used in this procedure
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| Health Certificate To Eu | Application For The Issue Of Veterinary Certificate To EU | 24-05-2018 | 05-06-2018 |
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Health Certificate for EU countries | Permit Requirement | Department of Fisheries and aquatic resources issues a Health Certificate for EU countries | Requirenment 1.The processing establishment should appear on the current list of EU approved establishments 2.The type of fish product should be within the scope of fish products for which the processing establishment has been approved 3.There should be no major non-conformities reported unattended, which are likely to affect the safety of fish products. 4.The establishment should not be under suspension by the Competent Authority or authorities of the market countries | Fisheries and Aquatic Resources Act | 31-12-9999 | Good |