View Procedure
| Procedure Name | The Registration of Medical Device | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Description |
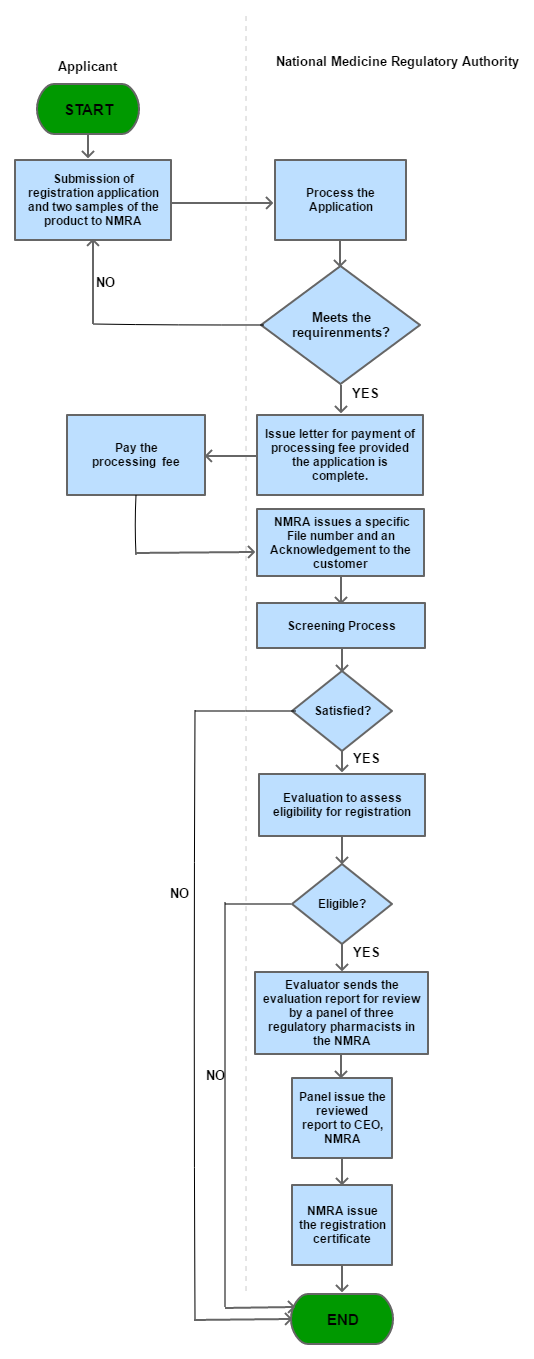
Process Steps
*This information may be subject to change in the future by the NMRA
| ||||||||||||||||||||||||||||||||
| Category | Import |
The following form/s are used in this procedure
| Title | Description | Created Date | Updated Date | Issued By |  |
|---|---|---|---|---|---|
| SCHEDULE I- Information required for registration of a device. | SCHEDULE I- Information required for registration of a device. | 05-07-2018 | 05-07-2018 |
This procedure applies to the following measures
| Name | Measure Type | Agency | Description | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|
| Registration of medical Device | Registration Requirement | Standard Operating Procedure For Registration Of Medical devices | The basis for the regulatory control comes from National Medicines Regulatory Authority Act, No. 5 Of 2015 | National Medicines Regulatory Authority Act | 31-12-9999 | Good |