View Procedure
| Procedure Name | The Registration of Borderline products |
|---|
| Description |
|
Category
|
Permit/license
|
|
Responsible Agency
|
Department of Import and Export Control
Address: 120, Norris Canal Road, Colombo 10, Sri Lanka
Phone: +94 11 2698896/7
|
|
Legal base of the Procedure
|
National medicines regulatory authority act, no. 5 of 2015
|
Required Documents
|
No.
|
Document type
|
|
1
|
Application
|
|
2.
|
Sample Import licence
|
|
3
|
Certification of Analysis for a finish products
|
|
4
|
Copy of free sale certificate issued by the health authority of the country of origin/Copy of COPP/ or a copy of certificate to prove registration status in country of origin
|
|
5
|
Classification form issued by NMRA with the approved formulation
|
|
6
|
Original Product Information and promotional material |
| 7 |
Packaging Material |
| 8 |
Check List |
Process Steps
|
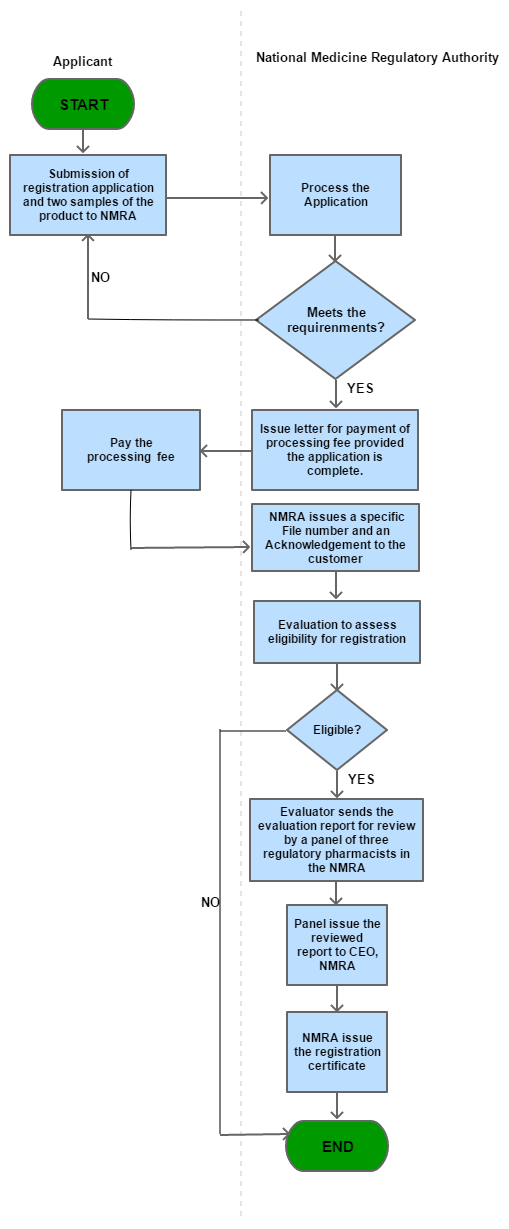
Step 1
|
Submission of registration application and two samples of the product to NMRA
|
|
Step 2
|
Process the Application
|
|
Step 3
|
Issue letter for payment of processing fee provided the application is complete.
|
|
Step 4
|
Pay the fee
|
| Step 5 |
NMRA issues a specific File number and an Acknowledgement to the customer |
| Step 6 |
Evaluation to assess eligibility for registration |
| Step 7 |
Evaluator sends the evaluation report for review by a panel of three regulatory pharmacists in the NMRA |
| Step 8 |
Panel issue the reviewed report to CEO, NMRA |
| Step 9 |
NMRA issue the registration certificate |
*This information may be subject to change in the future by the NMRA
|
|---|
| Category | Import |
|---|
The following form/s are used in this procedure
This procedure applies to the following measures


